Reinwarth, Michael ; Avrutina, Olga ; Fabritz, Sebastian ; Kolmar, Harald (2024)
Fragmentation follows structure: top-down mass spectrometry elucidates the topology of engineered cystine-knot miniproteins.
In: PLOS ONE, 2014, 9 (10)
doi: 10.26083/tuprints-00027627
Article, Secondary publication, Publisher's Version
| Item Type: | Article |
|---|---|
| Type of entry: | Secondary publication |
| Title: | Fragmentation follows structure: top-down mass spectrometry elucidates the topology of engineered cystine-knot miniproteins |
| Language: | English |
| Date: | 20 August 2024 |
| Place of Publication: | Darmstadt |
| Year of primary publication: | 2014 |
| Place of primary publication: | San Francisco, California, US |
| Publisher: | PLOS |
| Journal or Publication Title: | PLOS ONE |
| Volume of the journal: | 9 |
| Issue Number: | 10 |
| Collation: | 10 Seiten |
| DOI: | 10.26083/tuprints-00027627 |
| Corresponding Links: | |
| Origin: | Secondary publication service |
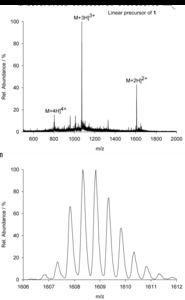
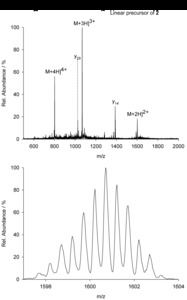
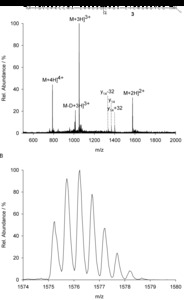
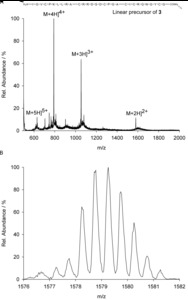
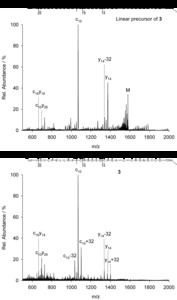
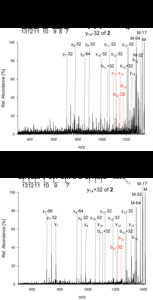
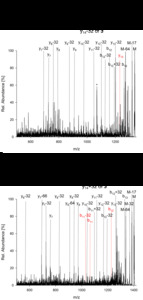
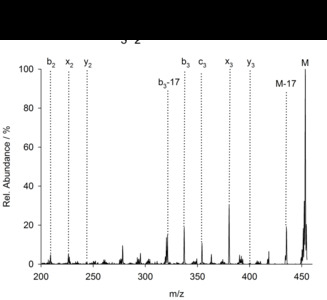
| Abstract: | Over the last decades the field of pharmaceutically relevant peptides has enormously expanded. Among them, several peptide families exist that contain three or more disulfide bonds. In this context, elucidation of the disulfide patterns is extremely important as these motifs are often prerequisites for folding, stability, and activity. An example of this structure-determining pattern is a cystine knot which comprises three constrained disulfide bonds and represents a core element in a vast number of mechanically interlocked peptidic structures possessing different biological activities. Herein, we present our studies on disulfide pattern determination and structure elucidation of cystine-knot miniproteins derived from Momordica cochinchinensis peptide MCoTI-II, which act as potent inhibitors of human matriptase-1. A top-down mass spectrometric analysis of the oxidised and bioactive peptides is described. Following the detailed sequencing of the peptide backbone, interpretation of the MS³ spectra allowed for the verification of the knotted topology of the examined miniproteins. Moreover, we found that the fragmentation pattern depends on the knottin’s folding state, hence, tertiary structure, which to our knowledge has not been described for a top-down MS approach before. |
| Uncontrolled Keywords: | Arginine, Disulfide bonds, Signal peptides, Cysteine, Amino acid analysis, Proteases, Protein structure, Ionization |
| Status: | Publisher's Version |
| URN: | urn:nbn:de:tuda-tuprints-276271 |
| Classification DDC: | 500 Science and mathematics > 540 Chemistry 500 Science and mathematics > 570 Life sciences, biology |
| Divisions: | 07 Department of Chemistry > Clemens-Schöpf-Institut > Fachgebiet Biochemie |
| Date Deposited: | 20 Aug 2024 13:25 |
| Last Modified: | 11 Sep 2024 06:57 |
| URI: | https://tuprints.ulb.tu-darmstadt.de/id/eprint/27627 |
| PPN: | 521306612 |
| Export: |
 |
View Item |


![[img]](https://tuprints.ulb.tu-darmstadt.de/27627/3.hassmallThumbnailVersion/Figure_S1.png)

![[img]](https://tuprints.ulb.tu-darmstadt.de/27627/4.hassmallThumbnailVersion/Figure_S2.png)
![[img]](https://tuprints.ulb.tu-darmstadt.de/27627/5.hassmallThumbnailVersion/Figure_S3.png)

![[img]](https://tuprints.ulb.tu-darmstadt.de/27627/6.hassmallThumbnailVersion/Figure_S4.png)
![[img]](https://tuprints.ulb.tu-darmstadt.de/27627/7.hassmallThumbnailVersion/Figure_S5.png)
![[img]](https://tuprints.ulb.tu-darmstadt.de/27627/8.hassmallThumbnailVersion/Figure_S6.png)
![[img]](https://tuprints.ulb.tu-darmstadt.de/27627/9.hassmallThumbnailVersion/Figure_S7.png)

![[img]](https://tuprints.ulb.tu-darmstadt.de/27627/10.hassmallThumbnailVersion/Figure_S8.png)
![[img]](https://tuprints.ulb.tu-darmstadt.de/27627/11.hassmallThumbnailVersion/Figure_S9.png)

![[img]](https://tuprints.ulb.tu-darmstadt.de/27627/12.hassmallThumbnailVersion/Figure_S10.png)

![[img]](https://tuprints.ulb.tu-darmstadt.de/27627/13.hassmallThumbnailVersion/Figure_S11.png)
![[img]](https://tuprints.ulb.tu-darmstadt.de/27627/14.hassmallThumbnailVersion/Figure_S12.png)
![[img]](https://tuprints.ulb.tu-darmstadt.de/27627/15.hassmallThumbnailVersion/Figure_S13.png)
 Drucken
Drucken
 Impressum
Impressum